Adsorption Dynamics
Research Topics: Adsorption Dynamics
Marangoni Effects in Surfactant Solutions
Surfactants adsorb at surfaces and lower the surface tension. If the adsorption is not uniform everywhere, surface tension gradients arise and cause flows in the bulk liquid adjacent to the surface. These Marangoni effects are important in stabilising foams, in spreading of droplets on solid surfaces, in the coalescence of droplets and in the break up of jets.
We have developed two platforms for studying Marangoni effects under steady-state hydrodynamic conditions: the overflowing cylinder (OFC) [1] and the axisymmetric liquid jet.[2,3] The surface age varies from 1 ms to 1 s. Laser Doppler scattering is used to measure the hydrodynamics and ellipsometry to measure the surfactant adsorption.
 |  |
Addition of surfactants to an overflowing cylinder can cause the surface velocity to increase by an order of magnitude. Intriguingly, the surface determines its own behaviour, independent of macroscopic parameters such as the bulk flow rate or the radius of the cylinder. Marangoni effects tend to be largest at bulk concentrations where there is appreciable adsorption to the dynamic interface, but the interface is not saturated by surfactant. Mass transport to the surface is critically important in determining the rate of surface expansion in the OFC, and features such as slow micellar diffusion, micellar breakdown or adsorption barriers can lead to large Marangoni effects even at high surfactant concentrations.
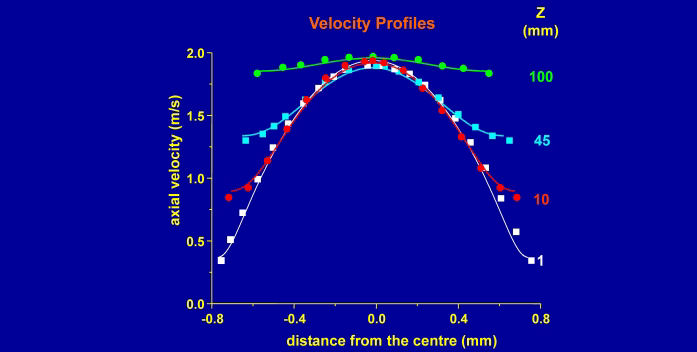
In the liquid jet, Marangoni effects are smaller than in the OFC, but large enough that they cannot be neglected in fluid dynamical models. Additionally, the fluid dynamics near the nozzle are fundamentally altered by the presence of surfactants, with the surface velocity scaling as the axial distance, z, from the nozzle rather than the usual z1/3 scaling.[4]
Adsorption Kinetics of Surfactants at the Air-Water Interface
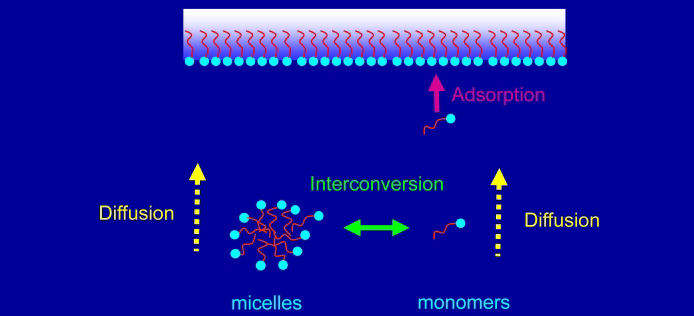
There are two traditional ways of studying adsorption kinetics of surfactants to the air-water interface. The first is to generate a fresh interface and observe the relaxation of the surface tension with time. The second is to create a surface at equilibrium and observe the response in the surface tension to small oscillations in area. We have adopted an alternative approach in which a non-equilibrium surface is generated in a flowing solution under steady-state conditions, using either an overflowing cylinder or a liquid jet. The mass transport equations are then solved at steady-state in the presence of convection. The advantages of this approach is the hydrodynamics are well-defined and that the amount of adsorbed surfactant can be measured directly by techniques such as ellipsometry, neutron reflection or FTIR. The equivalent surface age is in the range of 1 ms � 1 s. This methodology is being applied to pure surfactants [5,6] and mixed surfactants. Novel results include the observation of direct adsorption of micelles to the air-water interface, contrary to established models that assume that micelles must first decompose into monomers.[7]
Adsorption Kinetics of Surfactants at the Solid-Water Interface
We have developed a new methodology for measuring the kinetics of adsorption of surfactants to solid surfaces in water. A channel-flow cell is used to generate well-defined hydrodynamic conditions and ellipsometry to measure the amount of adsorption as a function of time. Check out a current student’s description of the project by following THIS LINK. Finite difference modelling is used to compute the surface coverage as a function of time and the predictions are compared with experimental results. This work is supported by Syngenta Ltd.
References
1. S. Manning-Benson, C. D. Bain and R. D. Darton “Measurement of Dynamic Interfacial Properties in an Overflowing Cylinder by Ellipsometry” Journal of Colloid and Interface Science 1997, 189, 109-116 (DOI).
2. T. Battal, C. D. Bain, M. Weiss and R. C. Darton “Surfactant Adsorption and Marangoni Flow in Liquid Jets. I. Experiments” Journal of Colloid and Interface Science 2003, 263, 250-260 (DOI).
3. D. M. Colegate and C. D. Bain “Marangoni Effects in Liquid Jets of Non-Ionic Surfactants” Australian Journal of Chemistry 2005, 58, 678-682 (DOI).
4. M. Weiss, R. C. Darton, T. Battal and C. D. Bain “Surfactant Adsorption and Marangoni Flow in Liquid Jets. 2. Modeling” Industrial & Engineering Chemistry Research 2004, 43, 5203-5220 (DOI).
5. D. S. Valkovska, G. C. Shearman, C. D. Bain, R. C. Darton and J. Eastoe “Adsorption of Ionic Surfactants at an Expanding Air�Water Interface” Langmuir 2004, 20, 4436-4445 (DOI).
6. M. Sekine, R. A. Campbell, D. S. Valkovska, J. P. R. Day, T. D. Curwen, L. J. Martin, S. A. Holt, J. Eastoe and C. D. Bain “Adsorption Kinetics of Ammonium Perfluorononanoate at the Air-Water Interface” Physical Chemistry Chemical Physics 2004, 6, 5061�5065 (DOI).
7. D. M. Colegate and C. D. Bain “Adsorption Kinetics in Micellar Solutions of Nonionic Surfactants” Physical Review Letters 2005, 95, 198302/1-4 (DOI).
8. Yang, L.; Bain, C. D. “Liquid Jet Instability and Dynamic Surface Tension Effect on Breakup” NIP 25: International Conference on Digital Printing Technologies and Digital Fabrication, 2009, 79-82. (PDF)
