Tensiometry
Techniques: Tensiometry
Introduction
Although many different techniques have been developed for the determination of the interfacial tension between two phases, they can all be grouped into four categories dependent upon the physical principles they exploit.
- Force methods, such as the Wilhelmy plate and du Noüy ring, measure the force exerted on an object suspended at the interface of interest. This force is related to the interfacial tension by a set of equations determined by the geometry of the experiment.
- Capillary wave methods, such as Surface Light Scattering, measure the frequency of light scattered from the thermally excited capillary waves which exist at all liquid-fluid interfaces. The frequency of the waves is related to the interfacial tension, as well as to the viscosity of the two fluid phases.
- Pressure methods, such as the Maximum Bubble Pressure technique, measure the balancing pressure difference across a curved interface that arises as a consequence of the interfacial tension.
- Drop Shape methods, such as pendant/rising and spinning drop techniques, utilise the balance between the forces which tend to distend the drop and the force due to interfacial tension which tends to contract the drop into a sphere.
Drop Shape Analysis
The principal method of tensiometry we use is Drop Shape Analysis, using either pendant drops or rising bubbles. The basic setup of the experiment is shown below.
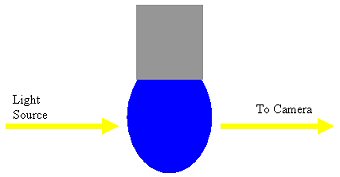
The droplet is constrained to a needle and is illuminated by a light source which projects the image of the drop onto a camera. The camera is connected to a computer allowing easy analysis of the image, via iterative fitting of the shape of the drop which is affected by the gravitational/buoyancy and interfacial tension forces acting on the drop.
In my group, dynamic interfacial tension measurements have been used to determine equations of state and adsorption isotherms for a range of surfactants through the use of the Gibbs equation, which allows the conversion of surface tension data to surface excess concentrations of surfactant at the interface.
Using equilibrium interfacial tension measurements as a function of temperature, surface freezing at the CTAB�tetradecane interface was studied. As shown below, the transitions are identified from kinks in the interfacial tension plots. The surface freezing temperature was found to vary linearly with the surface excess of CTAB.

