Raman Spectroscopy
Techniques: Raman Spectroscopy
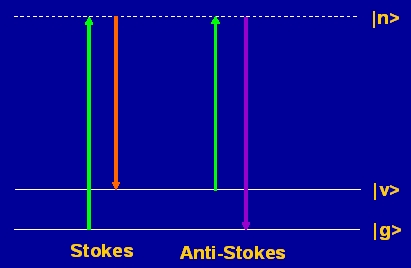
Raman spectroscopy is form of inelastic light scattering in which the scattered light gains or loses one quantum of energy from vibrational or rotational modes in molecules (or phonons in solids). Stokes scattering, in which the emitted photon has lower energy than the incident photon, is usually more intense than anti-Stokes scattering, owing to the Boltzmann distribution of populations over quantum states: anti-Stokes scattering requires the molecule initially to be in an excited state. In condensed phases, discrete rotational levels rarely exist and broad (several cm�1) vibrational transitions are observed. The selection rule for Raman scattering is that the polarisability must change during the vibration. It is complementary to infrared spectroscopy, for which the dipole moment must change. For centrosymmetric molecules, vibrations can be IR or Raman-active, but not both.
Raman spectroscopy is a weak effect (cross-section typically 10�29 cm2, compared to 10�16 cm2 for a fully allowed electronic transition) and special techniques are required to detect monolayers. One of these involves the use of surface plasmon excitation in roughened metal films to enhance the electric field near the surface. A second uses UV light in resonance with electronic transitions in the molecule. Neither of these methods provides a general approach to the detection of surfactants and lipids at interfaces.
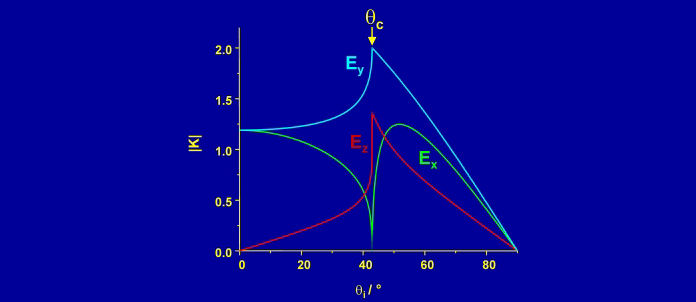
Raman spectroscopy lacks intrinsic surface sensitivity, but a high degree of surface selectivity can be achieved through the use of evanescent waves to confine the electric field to a region of space near an interface. If light is incident on the interface from an optically more dense to a less dense medium, there is a critical angle above which the light is totally reflected from the interface and only a rapidly decaying (evanescent) field exists in the less dense medium. For the solid-water interface, total internal reflection (TIR) can be used to confine the electric field to within a fraction of a wavelength of the surface. Water is a weak Raman scatterer, and monolayers of surfactants on solids can readily be distinguished from the water background.
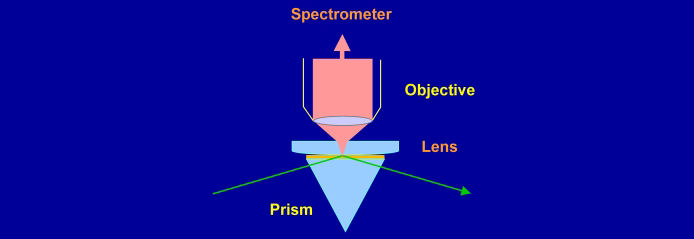
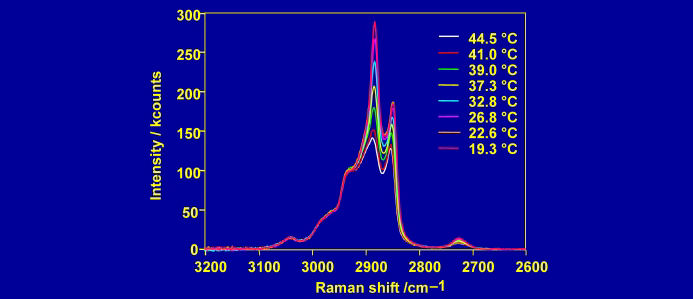
The electric fields (and hence the intensity of the Raman spectrum) are maximised at the critical angle. At this angle, the electric field is polarised perpendicular to the surface for p-polarised light and parallel to the surface for s-polarised light. The purity of polarisation is an asset for studying the orientation of molecules at an interface.